Key Inclusions
- A general overview of the viral clearance and testing studies, highlighting details on viral contamination in biologics and the need for viral clearance and testing. It also presents information on the process of viral clearance and testing, and regulatory guidelines related to viral contamination. Additionally, it features a discussion on the future perspectives of the viral clearance and testing industry.
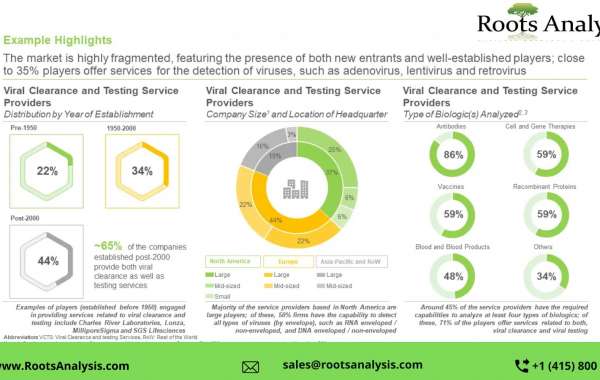
- A detailed assessment of the overall market landscape of the companies offering viral clearance and testing services, based on several relevant parameters, such as year of establishment, company size (in terms of number of employees), location of headquarters, location of viral clearance and testing facilities, type of virus detected (by envelope - RNA enveloped, RNA non-enveloped, DNA enveloped and DNA non-enveloped), type of virus detected (by class - retrovirus, adenovirus, lentivirus, adeno-associated virus (AAV), herpesvirus and others), key offerings (viral clearance services, viral testing services and viral clearance products), type of biologic(s) analyzed (antibodies, cell and gene therapies, vaccines, recombinant proteins, blood and blood products, hormones and others), method(s) of viral clearance (inactivation - pH treatment, solvent detergent, heat pasteurization and others), method(s) of viral clearance (removal - chromatography, filtration, precipitation and others) and type of viral testing service(s) offered (cell bank testing, raw material testing, unprocessed bulk testing and end of production process testing).
- A detailed competitiveness analysis of viral clearance and testing service providers based in North America, Europe and Asia-Pacific. The analysis compares the service providers based on supplier strength (in terms of years of experience and company size) and service strength (considering key offerings, type of virus detected (by envelope), type of virus detected (by class), method(s) of viral clearance (removal), method(s) of viral clearance (inactivation), viral testing service(s) offered, type of biologic(s) analyzed and additional services offered).
- Tabulated profiles of key players based in North America, Europe and Asia-Pacific that are engaged in providing services related to viral clearance and testing (shortlisted based on strength of service portfolio). Each profile includes a brief overview of the company, financial information (if available), information on viral clearance and testing related services, recent developments and an informed future outlook.
- An in-depth analysis of various patents that have been filed / granted for viral clearance and viral testing, since 2017, based on various relevant parameters, such type of patent, publication year, application year, type of applicant, emerging focus areas, patent jurisdiction, CPC symbols, leading players (in terms of number of patents granted / filed), patent benchmarking (in terms of CPC codes and leading players), patent characteristics and patent age. It also includes an insightful patent valuation analysis and list of leading patents by number of citations.
- A detailed analysis of recent developments taking place in this domain. This chapter includes the partnerships inked between stakeholders engaged in this domain, since 2015, covering instances of mergers and acquisitions, service agreements, service alliances, technology assessment agreements and other agreements. Further, it comprises of analysis of the deals inked based on year of partnership, type of partnership, purpose of partnership, type of organization, location of facility, and geography. It also highlights the most active players (in terms of number of partnerships) in the domain. In addition, this chapter also includes a detailed assessment of the recent expansion initiatives undertaken by various service providers to enhance viral clearance and testing capabilities, based on various parameters, including year of expansion, type of expansion, focus of expansion,
type of service(s) offered, type of biologic(s) involved, and geography. It also highlights the most active players (in terms of number of expansions) in the domain.
The financial opportunity within the viral clearance and testing services market has been analyzed across the following segments:
- Scale of Operation
- Discovery Phase
- Preclinical Phase
- Clinical Phase
- Method of Viral Clearance and Testing
- Viral Detection
- Viral Inactivation
- Viral Removal
- End-User
- Biotechnology and Pharmaceutical Companies
- Academic / Research Institutes
- Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Rest of the World
Key Questions Answered
- What is viral contamination in the biopharmaceutical industry?
- How many players are providing services related to viral clearance and testing?
- How many patents related to viral clearance and testing have been filed / granted in the last few years?
- What is the partnership and collaboration trend in the viral clearance and testing services domain?
- Which segment is likely to have the largest share in the viral clearance and testing services market?
- What are the recent developments in the viral clearance and testing services market?
- Which geographical segment has the highest growth rate in the viral clearance and testing services market?
To view more details on this report, click on the link
https://www.rootsanalysis.com/reports/viral-clearance-and-testing-services-market.html
You may also be interested in the following titles:
Non-Viral / Intracellular Drug Delivery Systems Market
Lipid Nanoparticles in Drug Delivery
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415